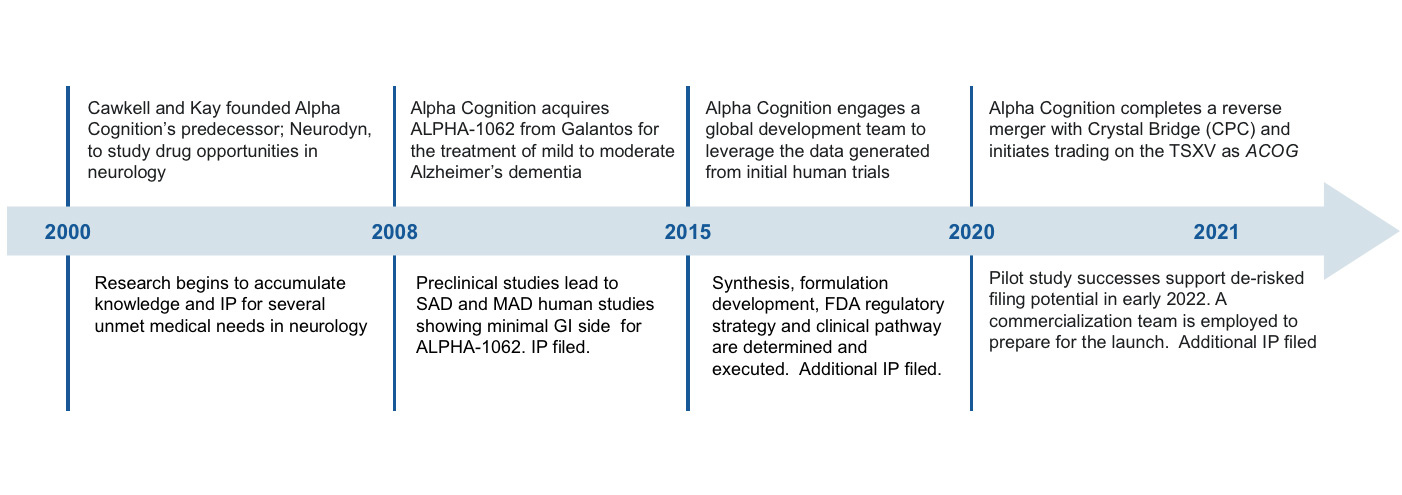
Alpha Cognition is a biopharmaceutical company dedicated to developing treatments for patients suffering from neurodegenerative diseases, such as Alzheimer’s disease for which there are limited or no treatment options. On July 26, 2024, the Company received approval by the FDA of the Company’s New Drug Application (the “NDA”) for ZUNVEYL® (benzgalantamine) previously known as ALPHA-1062 oral tablet formulation for the treatment of mild-to-moderate Alzheimer’s disease. The Company will now focus on the development of commercial manufacturing and commercial sales of ZUNVEYL oral tablet formulation.
The Company has three additional pre-clinical development programs: ZUNVEYL in combination with memantine for the treatment of moderate-to-severe Alzheimer’s disease, ALPHA-1062 sublingual formulation, ALPHA-1062 intranasal (“ALPHA-1062IN”) formulation for the treatment of cognitive impairment with mild traumatic brain injury (mTBI; otherwise known as concussion).